With the increase in population over the last few decades, the food industry has developed accordingly to satisfy the growing demands and feed the growing population. Technological advances and increased involvement of machines and robotics in food production has brought about many fold increase in the production of food. With the increase in food production, there is a growing danger of contamination by food pathogens. The three most common pathogens that cause food poisoning are E-coli, Salmonella and Listeria. The following are some examples of outbreaks for each of them:
Listeria outbreak caused by contaminated cantaloupes in 2011 caused 29 deaths1.
Salmonella outbreak in 2008-2009 due to peanut paste and peanut paste facilities owned by Peanut Corporation of America affected 46 states and Canada resulting 714 cases of food poisoning1.
E-coli outbreak involving bagged spinach in 2006 affecting 22 states, 238 infection and 5 deaths1.E-coli infection of Romaine lettuce in Minnesota affecting 40 people from 16 states2.
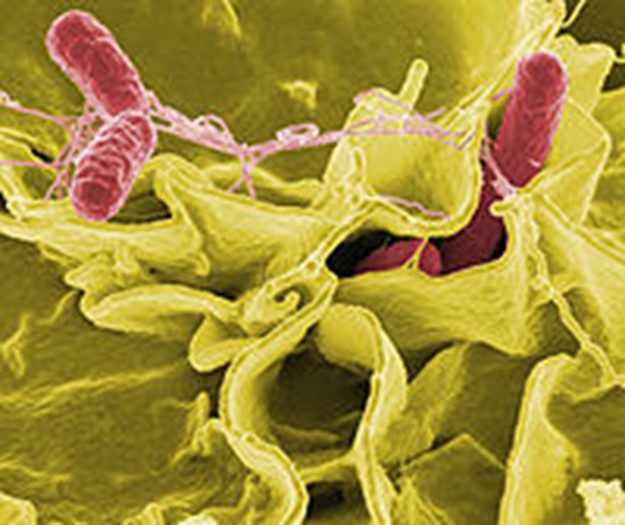
Figure 1: Salmonella in food
.Figure 2 is a depiction of the romaine lettuce E-coli bacteria O175 infection originated in Salinas, California2

Figure 2: E-coli O157 contamination of romaine lettuce was originated in Salinas California in 20192
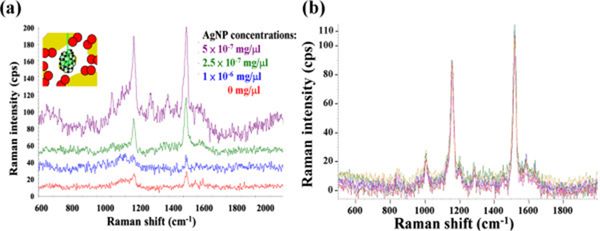
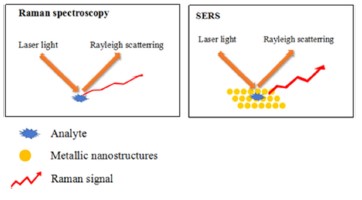
Spectroscopy has been a useful tool in identification of food pathogens. Both IR and Raman spectroscopy techniques have been used to identify food pathogens. Raman spectroscopy uses the inelastic scattering from molecules as a finger print to identify the molecule. Stokes and anti-stokes lines represent the scattered lower and higher frequencies respectively. Raman is a real-time method that is capable of quickly and effectively identifying the bio-molecules with a variety of chemical structures and chemical compositions. Figure 3 shows a typical Raman spectra from a biomolecule.
However, Raman spectroscopy has certain limitations which makes it challenging to identify bacteria as follows:
Raman signals are generally weak and to generate them, a laser is needed.The intensity of Raman signal is weaker by a factor of 108 with respect to the source signal.
The fluorescence signal often interferes with Raman and needs to be suppressed.
The equipment cost is often high because there is always a need for a laser source. Visible laser sources such as Argon lasers are expensive to purchase.
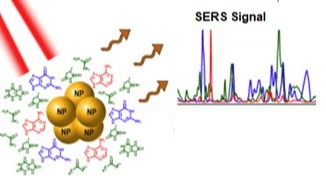
The colloid method is based on the use of noble metal gold nano-particles which have diameters between 10 to 200 nm. Gold colloid is a very effective technique to effectively identify pathogens through SERS. They are inexpensive, can be easily prepared and are easily available. Figure 5 shows a gold colloid in proximity of an organic sample and generation of the SERS signal7.
Figure5: Gold colloid usage for generation of SERS signal7
The metal nano-particle colloid method is inexpensive and easy to prepare but shows poor repeatability of the results. Another technique is to use nano-structured metal surface based SERS method4. In this technique a surface is prepared with clusters of nano-particles attached to it. Using this technique will bring about several advantages as follows:
There is good repeatability
Live and dead bacteria can be distinguished
Three types of bacteria can be found
There is a 108 increase in signal intensity
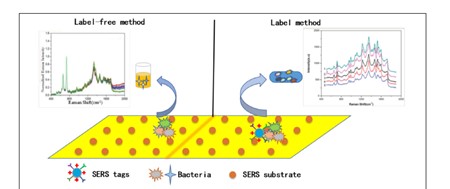
References:
3- Rapid identification of bacteria utilizing amplified dielectrophoretic force-assisted nano-particles-induced surface-enhanced Raman spectroscopy, I-Fang Cheng et al., Nano-scale research letters, June 2014.
4- Detection of foodborne pathogens by Surface Enhanced Raman Spectroscopy, X. Zhao et.al, Frontiers in microbiology, June 2018.
5- M. Fleischmann et al., Raman speold clusters ctra of pyridine adsorbed at a silver electrode, Chem. Phys. Lett. 26, 1974.
6- Surface Raman spectro electro chemistry: part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode, J. Electroanal. Chem. Interfacial Electrochem 84, 1977.
7- Colloidal gold clusters formation an chemometrics for direct SERS determination of bioanalytes in complex media, J.E.L. Villa et.al, Spectrochimica Acta Part A: Molecular and biomolecular spectroscopy, 224 (2020).